岛津GDX-101 2015版药典溶剂残留测定填充柱
简单介绍
岛津GDX-101 2015版药典溶剂残留测定填充柱的详细介绍
2015版药典溶剂残留测定填充柱 详细信息:
名称:填充柱
固定相:苯乙烯-二乙烯苯共聚物
规格:2m*3mm
粒度:60-80目
型号:GDX-101
仪器:岛津2014
应用:药典2015溶剂残留测定:甲磺酸左氧氟沙星中有机溶剂残留;头孢菌素类抗生素残留溶剂的测定
随着医药工业技术的提高以及人民健康水平的提高。各国药典对药物质量指标提出愈来愈高的要求和标准。
95版中国药典收入了气相色谱法测定有机溶剂残留量。但不同溶剂采用不同柱温条件测定,甚是麻烦。美国药典可同时测定多种有机溶剂残留,但条件苛刻,操作繁复。
为此,浩瀚色谱(山东)应用技术开发有限公司并研究提出了同时测定药物多种有机溶剂残留量的气相色谱法。测定条件是:GDX-101填充柱,柱温150℃,进样口温度210℃。气体流速氮气50ml/空气0.5kg/cm~2,氢气0.5kg/cm~2,检测器为氢火焰,量程10~3,数据处理机衰减值为,进样浓度按药典规定配制,进样量为3μl。结果表明:可以同时测定多种溶剂,方法简便、准确、快速、能满足药典要求。
2015版药典溶剂残留测定填充柱 测试谱图:




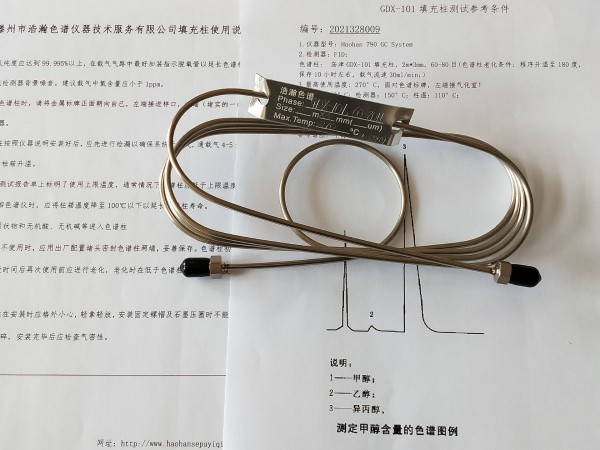
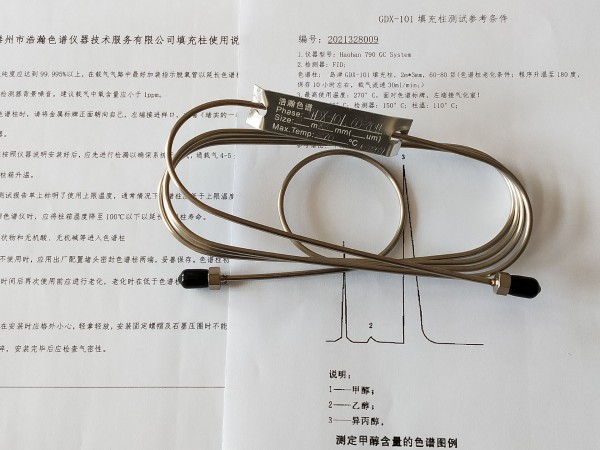


Packed column for determination of solvent residue in Pharmacopoeia of 2015 edition
Packed column for determination of solvent residue in the 2015 Pharmacopoeia Details:
Name: Packed column
Statio
Specification: 2m*3mm
Granularity: 60-80 mesh
Model: GDX-101
Application: Pharmacopoeia 2015 solvent residue determination: organic solvent residue in levofloxacin mesylate; determination of cephalosporin antibiotic residue solvent
With the improvement of pharmaceutical industry technology and the improvement of people's health. Pharmacopoeias of various countries have put forward higher and higher requirements and standards for drug quality indicators.
The 95th edition of the Chinese Pharmacopoeia includes gas chromatography for the determination of residual organic solvents. However, it is very troublesome to use different column temperature co
To this end, Haohan Chromatography (Shandong) Applied Technology Development Co., Ltd. has also researched and proposed a gas chromatography method for simultaneous determination of multiple organic solvent residues of drugs. The measurement co
Test spectrum of packed column for determination of solvent residue in Pharmacopoeia of 2015 edition:
相关产品
在线留言 |










