Porapak Q 填充柱测定乙醛残留 YBB00102002 口服液体药用聚酯瓶(试行)
产品名称:Porapak Q 填充柱测定乙醛残留 YBB00102002 口服液体药用聚酯瓶(试行)
产品型号:Porapak Q 填充柱测定乙醛残留
产品厂商:浩瀚色谱(山东)应用技术开发有限公司
简单介绍
Porapak Q 填充柱测定乙醛残留 YBB00102002 口服液体药用聚酯瓶(试行)的详细介绍
YBB00102002 口服液体药用聚酯瓶(试行) 详细信息:
名称:填充柱
固定相:乙基乙烯苯-二乙烯苯共聚物
规格:2m*3mm
型号:Porapak Q
粒度:60-80目
应用:YBB00102002-2015口服液体药用聚酯瓶
YBB00102002 口服液体药用聚酯瓶(试行)
本标准适用于以聚对苯二甲酸乙二醇酯( PET) 为主要原料 ,采用注吹成型工艺生产的口服液体制剂用塑料瓶 。
【 乙醛】 照乙醛测定法 ( YBB00282004-2015 ) 第yi法测定,不得过千万分之二 。
浩瀚色谱(山东)应用技术开发有限公司建立口服液体药用聚酯瓶中乙醛残留量的测定方法。方法:采用顶空气相色谱法。色谱柱为Porapak Q填充柱,氢火焰离子化检测器,进样口温度为200℃,检测器温度为200℃,载气为氮气;顶空进样器平衡温度为70℃,平衡时间为30min,程序升温。结果:乙醛检测浓度线性范围为0.002~0.02mg·L-(1r=0.9991),平均回收率为98.37%,RSD=2.82%(n=6),检测限为0.1ng。结论:本方法准确,可用于口服液体药用聚酯瓶中乙醛的限度检查。
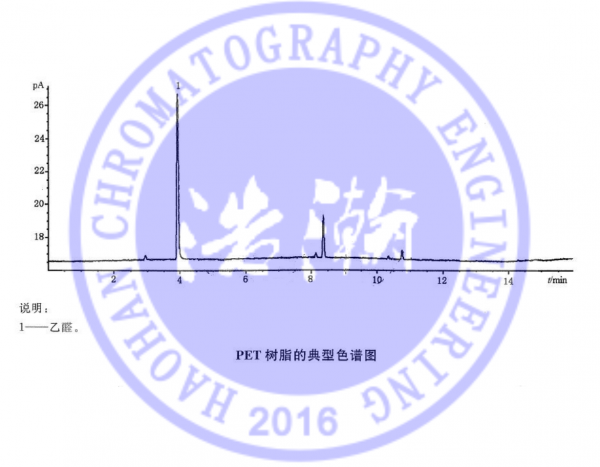
YBB00102002 口服液体药用聚酯瓶(试行) 测试谱图:





YBB00102002 Oral liquid medicinal polyester bottle (trial)
YBB00102002 Oral liquid medicinal polyester bottle (trial) Details:
Name: Packed Column
Statio
Specifications: 2m*3mm
Model: Porapak Q
Granularity: 60-80 mesh
Application: YBB00102002-2015 Oral liquid medicinal polyester bottle
YBB00102002 Oral liquid medicinal polyester bottle (trial)
This standard applies to plastic bottles for oral liquid preparations produced by injection blow molding with polyethylene terephthalate (PET) as the main raw material.
【Acetaldehyde】According to the acetaldehyde determination method (YBB00282004-2015) yi method, it should not exceed 2/10 million.
Haohan Chromatography (Shandong) Application Technology Development Co., Ltd. has established a method for the determination of acetaldehyde residues in oral liquid pharmaceutical polyester bottles. Methods: Headspace gas chromatography was used. The chromatographic column was a Porapak Q packed column, a hydrogen flame io
YBB00102002 Oral liquid medicinal polyester bottle (trial) Test spectrum:
相关产品
在线留言 |










